Introduction:
Venous thromboembolism (VTE) is one of the major causes of mortality among patients with malignancy. It is estimated that around 4-20% of patients with cancer experience venous thrombosis. Typically, low molecular weight heparin (LMWH) is used as an initial agent of choice in VTE except in patients with renal insufficiency who cannot tolerate LMWH and require alternate therapy. Also, despite adequate therapy, VTE recurrence is common among cancer patients. The novel oral anticoagulants (NOACs) have recently been introduced in the treatment of VTE and have shown promising results. We have performed a systemic review of the literature comparing the safety and efficacy profiles of NOACs with LWMH in cancer patients with VTE.
Methods:
We performed a comprehensive literature search completed on July 7, 2020, on Pubmed, Cochrane library, and ClinicalTrials.gov. We used the MeSH terms: 'venous thrombosis', 'neoplasms', 'rivaroxaban', 'dabigatran', and 'enoxaparin' with associated entry terms. Our search yielded 46 studies. Following PRISMA guidelines and subsequent screening by three reviewers, we shortlisted 5 completed clinical trials (n=2873) and included data from these studies in our systemic review.
Results:
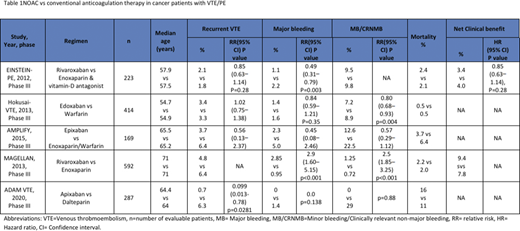
EINSTEIN-PE investigators (2013, n=223) reported a net clinical benefit (VTE plus major bleeding) in 83 patients (3.4%) vs 96 patients (4%) in the rivaroxaban arm versus control arm (enoxaparin + warfarin/acenocoumarol arm) respectively (HR 0.85 [95% CI 0.63-1.14], p=0.28). Recurrence occurred in 2.1% vs 1.8% (HR 1.12 [95% CI 0.75-1.68] p=0.003). Major bleed events occurred in 1.1% vs 2.2% (HR 0.49 [95% CI 0.31-0.79] p=0.003). Clinically relevant minor bleed events occurred in 9.5% vs 9.8%. Deaths were lower in rivaroxaban arm (20 vs 23).
Hokusai-VTE investigators (2013, n=414) reported edoxaban as non-inferior to warfarin when compared the primary efficacy (defined as recurrence of symptomatic VTE) 3.2% vs 3.5% respectively (HR 0.89 [95% CI 0.70 to 1.13] p<0.001). Recurrence occurred in 3.4% vs 3.3% (HR 1.02 [95% CI 0.75-1.38]). Major bleed events occurred in 1.4% vs 1.6% (HR 0.84 [0.59-1.21] P=0.35). Non-major bleed events were significantly lower in edoxaban arm 7.2% vs 8.9% (HR 0.80 [0.68-0.93] p=0.004). 20 participants died in edoxaban arm vs 21 in control.
AMPLIFY study (2015, n=169) reported reduced recurrence rate of 3.7% vs 6.4% in apixaban vs conventional therapy (enoxaparin + warfarin) respectively (RR 0.56 [95% CI 0.13-2.37]). Major bleed events weremore common in controls 2.9% vs 5% (RR 0.45 [95% CI 0.08-2.46]). Non major bleed events occurred in 12.6% vs 22.5% (RR 0.57 [95% CI 0.29-1.12]). Three vs five deaths occurred in apixaban arm vs conventional therapy respectively.
MAGELLAN study (2013, n=592) reported a better net clinical benefit (defined as primary efficacy outcome up to 10 days or safety outcome up to 35 days) of 9.4% vs 7.8% in rivaroxaban vs enoxaparin respectively. Recurrence was low in rivaroxaban (4.8% vs 6.4%). Major bleed events occurred more commonly in rivaroxaban arm 2.85% vs 0.95% (RR 2.9 [95% CI 1.60-5.15] p<0.001). Non-major bleed events were more frequent with rivaroxaban 1.25% vs 0.72% (RR 2.5 [1.85- 3.25] p<0.001). Deaths occurred in 2.2% vs 2% in intervention vs control.
ADAM VTE (2020, n=300) reported recurrence in 0.7% vs 6.3% in apixaban vs dalteparin respectively (HR 0.099 [95% CI 0.013-0.78] p=0.0281). Major bleed events noted in 0 vs 1.4% (p=0.138) and clinically relevant non-major bleed events noted in 6.2% vs 4.2% patients in the apixaban vs control respectively. Deaths were more common in apixaban group 23 (16%) vs dalteparin group 15 (11%).
Conclusion:
NOACs have comparable efficacy to conventional LMWH based treatment options and are feasible alternate therapy for VTE in patients with cancer. Apixaban so far has demonstrated better efficacy profile among the NOACs. However, the data at present is conflicting regarding safety profile. Large double-blinded randomized clinical trials are required to confirm the safety profile.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.